 D
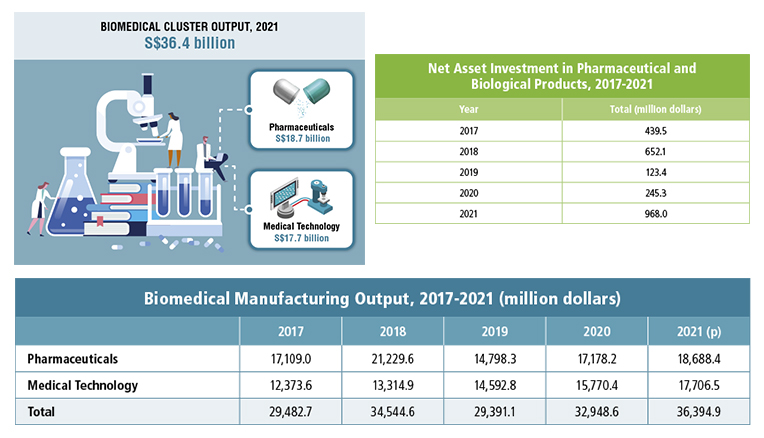
Driven by strong demand for vaccines, investment in Singapore’s biomedical industry has reached new levels. According to the Singapore Economic Development Board (EDB), Singapore drew S$968 million in biomedical net asset investment (NAI) in 2021, over 2.5 times more than the cumulative total for 2019 and 2020, accounting for over 11% of the Singapore’s manufacturing NAI.
Top on the list was Sanofi which is building a S$638 million state-of-the-art Evolutive Vaccines Facility (EVF) at Tuas Medical Park, its largest investment to date. Scheduled to come on stream in 2025, it is designed to produce multiple vaccines across different biological platforms, including mRNA, enzymes and monoclonal antibodies. This modularity and flexibility will allow the faster production of a vaccine when a public health crisis hits.
Speaking at the groundbreaking ceremony on 20 April 2022, Deputy Prime Minister and Coordinating Minister for Economic Policies Heng Swee Keat, noted, “Disease X is not a matter of if, but when. Yet no one knows what it would be like, nor when it could strike. But we know that vaccines will remain at the heart of how we deal with the threats of infectious diseases.”
“By enhancing our capacity for manufacturing vaccines in Singapore, the region will be in a stronger position for dealing with future pandemics and the ensuing economic shocks.”
Singapore’s strengths
Singapore ranks high on the list as manufacturing hub for pharmaceutical companies for good reasons. As Mr Thomas Triomphe, Executive Vice President, Head of Vaccines, Sanofi, told The Straits Times, “Singapore is not just an economic hub but also a technology and innovation hub. To proceed with massive investments like the EVF, you need to have a whole ecosystem of suppliers of raw materials, of starters, of innovation technologies in the same area.”
Already here are 60 manufacturing facilities producing a wide range of products, from antibiotics and flu vaccines to life-saving medicine for hypertension and diabetes. Tuas Biomedical Park, a dedicated hub in Singapore’s west, is home to 13 global biopharmaceutical companies.
Besides global multinational corporations, Singapore has a strong ecosystem of local enterprises, including cancer immunotherapy firm Tessa Therapeutics and precision therapy company Hummingbird Bioscience.
Equally important for global vaccine manufacturers is Singapore’s small population. As the production capacities of manufacturing facilities will easily exceed the local requirements, they can safely rule out any possibility that export controls may be enforced during a pandemic. With Singapore’s extensive air and sea connectivity and cold chain facilities, products manufactured here can be easily shipped across the region and beyond.
After donating or selling more than 66 million doses, India, the world’s largest vaccine manufacturer, withheld vaccine export for six months, April to September 2021, to inoculate its own population following a Covid-19 infection spike.
Since it was first promoted in 2000 Singapore’s pharmaceutical industry has posted strong gains. From S$4.8 billion in its first year, pharmaceutical manufacturing output rose to S$18.7 billion in 2021, lifting manufacturing output of the biomedical cluster to S$36.4 billion.
 Bumper profits from vaccines for pharma majors
Bumper profits from vaccines for pharma majors
It was a stellar year for industry majors, particularly companies with vaccines to combat Covid-19. Pfizer set the stage with a record-breaking performance. Net income surged 140% to US$22 billion in 2021 on US$81.3 billion in revenue, 95% higher than the previous year. If contributions of Comirnaty and Paxlovid were excluded, the revenue for 2021 would have been a more modest US$44.4 billion, up 6% over the previous year.
Comirnaty, which it jointly developed with German biotechnology company BioNTech, is the first FDA approved Covid-19 vaccine while Paxlovid is Pfizer’s oral antiviral treatment.
“We put billions of dollars of capital on the line in pursuit of those goals, not knowing whether those investments would ever pay off. Now, less than two years since we made that commitment, we are proud to say that we have delivered,” said Pfizer’s Chairman and Chief Executive Officer, Mr Albert Bourla.
The company expects more growth going forward, because of “durable Covid-19 revenue” amongst other things. For 2022, Pfizer is aiming to become the first pharmaceutical company to break the US$100 billion barrier in yearly revenue, with Comirnaty and Paxlovid accounting for half the total.
Roche also enjoyed a small boost from Covid-19 solutions, mainly from diagnostics. The Swiss manufacturer launched the world’s first commercially available Covid-19 PCR test two years ago and has since distributed ten million tests. Core operating profit (earnings before interest and tax, adjusted for one-off items) rose 2% to CHF21.9 billion on revenue of CHF62.8 billion in 2021, an increase of 8% over the previous year.
Pharma industry’s daily monitor Fierce Pharma noted, “Amazingly, in 2021 none of the top 20 companies had a revenue decrease.” In contrast in 2020, six companies in the top 20 posted a drop in revenue and only two had an increase greater than 10%, with gains due almost entirely to major acquisitions.
Of the top 20, BioNTech and Moderna recorded the biggest earnings leap in 2021. BioNTech’s revenue rose 3,835% to €19 billion while net profit increased by an astounding 67,614% to €10.3 billion. Following its success, the company has raised its R&D spend for 2022 to €1.4 billion - €1.5 billion, about 50% more than 2021.
“To continue our industry leadership, we intend to build on our 2021 success and rapidly advance multiple programmes, including our mRNA-based immunotherapies, cell therapies, and bi-specific antibodies. At the same time, we are investing in our second growth pillar, infectious diseases, and intend to advance our influenza and shingles vaccine candidates together with our partner Pfizer. In parallel, we also intend to invest heavily in regenerative medicine and autoimmune diseases with the aim to develop further therapeutic innovations addressing high unmet medical need. Our core vision remains the foundation for all our activities: harnessing the power of the immune system to improve the health and lives of billions of people worldwide,” said Mr Ugur Sahin, M.D., CEO and Co-Founder of BioNTech.
For Moderna, its Covid-19 vaccine, Spikevax, was a giant shot in the arm for the company lifting its revenue 2,200% to US$18.5 billion in 2021. Spikevax, the company’s only commercially available product, accounted for US$17.7 billion of 2021 sales. Net profits rose to US$12.2 billion in 2021 from a net loss of US$747 million in 2020.
Moderna CEO Stéphane Bancel said, “Spikevax is now approved in more than 70 countries around the world protecting hundreds of millions of people and real-world evidence from multiple independent studies has confirmed its strong effectiveness.”
“In 2021, we delivered 807 million doses with approximately 25% of those doses going to low- and middle-income countries, and we will continue to scale in 2022 to help end the Covid-19 pandemic.”
Steady increase in global spend on medicines
Driven by Covid-19 vaccinations, global spending on medicines is expected to trend up. The IQVIA Institute for Human Data Science projects that it will increase at a compound annual growth rate (CAGR) of 3-6% to reach US$1.8 trillion in 2026. The cumulative global spending on vaccines and therapeutics will account for a sizeable chunk of the total.
IQVIA noted, “The total cumulative spending on Covid-19 vaccines since their introduction through 2026 is projected to be US$251 billion, largely from the initial wave of vaccinations to be completed by 2022 in most countries. In subsequent years, booster shots are expected to be required annually or more often as the limited durability of immunity and the continued emergence of viral variants drive recommendations for additional inoculations.”
IQVIA expects another US$58 billion will be spent on therapeutics
With such massive sums channelled towards addressing the ill effects of Covid, non-Covid spending on medicines is expected to drop by US$175 billion over the same period. IQVIA estimates the net increase in global spending on medicine over the next five years to be US$133 billion.
Pharmerging markets led by China will drive spending growth over the forecast period rising by 2.5% to 5.5%. US, the world’s biggest market, is forecast to grow at 0 to 3% CAGR over the next five years, down from 3.5% CAGR in the previous five years, while Japan, the third biggest market, will have flat-to-declining medicine spending.
IQVIA expects 300 drugs to be launched during the period, significantly higher than levels seen during the past decade, skewed toward specialty, niche and orphan drugs. The two leading global therapy areas - oncology and immunology - are forecast to grow at 9-12% and 6-9% CAGR, respectively, lifted by significant increases in new treatments and medicine use and offset by the impact of biosimilars.
In neurology, many new medicines are expected across a range of diseases, including novel migraine therapies, potential treatments for rare neurological diseases and potential therapies for Alzheimer’s and Parkinson’s diseases.
Smaller-scale acquisitions and bolt-on
Biopharma mergers and acquisitions (M&As) for 2021 was one of the lowest in a decade. According to the annual EY M&A Firepower report, deals totalling US$108 billion were concluded, down from US$128 billion in 2020 and US$261 billion in 2019. It would have been far worst were it not for a flurry of deals in the last quarter.
Unlike the previous years, there was no mega deal. Rather the deals were mainly bolt-on acquisitions which were smaller in scale, aimed at bulking up R&D pipelines. Oncology was a key driver, as was rare disease and immunology/inflammation.
The largest deal involving Australia’s CSL’s acquisition of Switzerland’s Vifor Pharma for US$11.7 billion was announced in December. With the purchase, CSL, who is heavily dependent on vaccines and blood plasma products, will have a more diversified portfolio with the addition of ten commercialised products, including Ferinject/Injectafer, Venofer, Veltassa and Korsuva.
Size-wise, it was just ahead of Merck & Co.’s US$11.5 billion takeover of Acceleron Pharma aimed at strengthening its growing cardiovascular portfolio. The takeover gives Merck Acceleron’s lead clinical candidate sotatercept, a potential first-in-class therapy for pulmonary arterial hypertension, which has potential sales of US$2 billion toUS$3 billion.
Biopharma companies closed the year with near-record levels of firepower, the report noted. EY teams define firepower as a company’s capacity to do M&A based on the strength of its balance sheet. Only 9% of biopharma’s firepower was deployed on M&A in 2021, compared with 25% in 2019 and 12% in 2020.
Analysts expect that with the looming patent cliff, as nine of the industry’s top 20 drugs by sales are set to lose exclusivity over the coming years, there will be a surge in deals going forward. As PWC noted in its Pharmaceuticals and Life Sciences: Deals 2022 Midterm Outlook, “All of the stars are aligned for there to be a flurry of deals activity across all areas of the sector despite the slow start to the year so far. Many large pharma players are flush with cash (particularly those that have Covid-19 treatments in their arsenal), biotech valuations have been normalising after years of a boom market and the 2025 patent cliff is rapidly approaching, all making for a strong deal environment.”
But with increased scrutiny from the US Federal Trade Commission around larger deals, PWC expects that deals will mainly be bolt-on transactions in the US$5 to US$15 billion range, though it does not entirely rule out the potential for larger deals.
New FDA drug approvals
Fifty novel drugs received the US Food and Drug Administration (FDA) approval in 2021, in line with recent trends, despite the continued impact of Covid-19. Among them were AbbVie’s Qulipta for episodic migraine, Leo Pharma’s ADBRY for moderate to severe eczema, and Biogen’s costly Aduhelm, the first Alzheimer’s drug for cognitive decline, which debuted at a princely cost of US$56,000 a year.
Twenty-six of the drugs approved were for rare or orphan diseases defined as diseases that affect less than 200,000 people in the US. Among them was Besremi (ropeginterferon alfa-2b-njft) injection to treat adults with polycythemia vera, a blood disease that causes the overproduction of red blood cells. The excess cells thicken the blood, slowing blood flow and increasing the chance of blood clots.
FDA’s Center for Drug Evaluation and Research (CDER) also took steps to advance treatment options for patients with diabetes by approving several diabetes medications, including one interchangeable biosimilar insulin product and one biosimilar insulin product, which can provide patients with additional safe, high-quality, and potentially more cost-effective options. CDER also approved a treatment for paediatric patients ages 10 and older with type 2 diabetes and a therapy for severe hypoglycemia, or low blood sugar, in patients aged six years and older with diabetes.
Yet, some applications did face pandemic challenges. In November 2021, FDA reported that 55 new drug applications encountered inspection delays.
Record high for drug innovation
Drug innovation is setting new records in the level of investment, activity and scientific progress, in addition to the number and range of new medicines reaching patients in spite of the many operational and organisational challenges presented by Covid-19, said the IQVIA in its latest report, Global Trends in R&D: Overview through 2021.
In terms of new drug approvals and launches, 84 novel active substances (NASs) were launched globally in 2021, double the number five years ago, bringing the total over the past 20 years to 883. The US headed the field with 72 NASs launched in 2021, of which 44 were characterised by the FDA as first-in-class, and more than half carried an orphan drug designation.
R&D reached new levels. Over 6,000 products are on human trials globally, up 68% over 2016, as companies continued to invest and advance innovative therapeutics and vaccines across a wide range of disease areas. In oncology, targeted therapies account for almost all of the research, and over 40% of the pipeline was for rare cancers.
Overall clinical trial activity was sustained through the pandemic, IQVIA noted, as the industry adapted to the disruption and developed new approaches to enable research to continue. In 2021, 5,500 new planned clinical trial starts were reported, up 14% over 2020 and 19% higher than 2019.
Over 2 million study participants were involved in new trials for the first time in 2021, double the level seen prior to the trials for both Covid-19 and several very large Ebola virus vaccine trials.
As for funds committed to research, the 15 largest pharmaceutical companies invested a record US$133 billion in R&D expenditure in 2021, an increase of 44% since 2016. Venture capital funds were also available, especially in the US. They provided funding for over 2,000 deals totalling US$47 billion in deal value. Life sciences deal transactions – including licensing, collaborative R&D arrangements and acquisitions – remained at just under 5,000 deals in 2021, including over 500 related to Covid-19.
Emerging biopharma companies – those with an estimated R&D expenditure of less than US$200 million and annual revenue of less than US$500 million – were responsible for a record 65% of research, up from less than 50% in 2016 and one-third in 2001.
IQVIA further noted the innovation cycle time has accelerated, with the median time from first patent filing to launch in the US falling to its lowest level in the past two years. Among them were 21 drugs that were launched less than five years into their patent terms. Almost three-quarters received some form of expedited review by the FDA, including one in four receiving an accelerated approval or an Emergency Use Authorisation.
Going forward
The worst of the pandemic may well be behind us but Covid-19 has reinforced the importance of pandemic preparedness and vaccine development.
“Covid-19 has reinforced the importance of pandemic preparedness and supply chain resilience. We must not take our foot off the pedal when the pandemic fades,” said Mr Heng at Sanofi’s EVF groundbreaking ceremony.
Besides Sanofi, several other pharmaceutical companies are also ramping up their presence in Singapore as Covid-19 has strengthened the case for vaccine development for a host of diseases, including malaria, tuberculosis and HIV. Even though the pandemic caused 6 million deaths and the worst economic downturn in a century, it could have been a lot worst if it were not for the expeditious development of safe and effective vaccines. A century ago, at least 50 million lives worldwide were lost because of the Spanish Flu.
Hilleman Laboratories has set up a 9,144-sq. metre manufacturing plant in Depot Road to supply clinical trial materials for biologics and vaccines up to phase 2, and an R&D facility at Biopolis focusing on early product development of vaccines and biologics; pharmaceutical contract manufacturer Thermo Fisher Scientific expanded its Asia Pacific manufacturing capability with the addition of two sterile filling lines, including one large-scale line dedicated to live-virus filling; and BioNTech has plans for a manufacturing facility in Singapore to produce a range of novel mRNA vaccines and therapeutics for infectious diseases and cancer.
Until the pandemic hit, there was just one vaccine plant in Singapore which was set up by GlaxoSmithKline in 2011 to manufacture pneumococcal and Haemophilus influenzae antigens for the company’s childhood bacterial vaccines.
Also here is leading Contract Research, Development and Manufacturing Organization (CRDMO) WuXi Biologics. In July 2022, the Shanghai headquartered company announced a 10-year US$1.4 billion (S$2 billion) investment plan to expand the company’s research, development, and large-scale drug substance and drug product manufacturing capacity and capabilities in Singapore. Slated to be completed in 2026, the plant will add 120,000 litres of manufacturing capacity to its global network and employ 1,500 staff in research and production.
Currently, the company has over 10,000 employees in China, the United States, Ireland, Germany and Singapore.
Research is also being stepped up. Over five years to 2025, Singapore is committing S$25 billion to research, innovation and enterprise RIE2025 and advanced manufacturing, which includes biopharma production, is a priority area.
One programme is the Pharma Innovation Programme Singapore, or PIPS, a public-private consortium involving global pharma companies and researchers that aims to address industry challenges in small molecule manufacturing.
“PIPS has had early success in improving the process for continuous manufacturing,” said Mr Heng. “Building on this momentum, we are further expanding this initiative under RIE2025 to include biologics and vaccine manufacturers. A new programme – BIOPIPS – will be started to enable more companies to work on pre-competitive co-innovation.”
It is not a question of if the next pandemic will hit, but when. By ramping up capacity for manufacturing vaccines in Singapore, we can strengthen the region’s position to withstand future pandemics or supply chain shocks.
